We have published our paper entitled "Generation and primary characterization of iAM-1, a versatile new line of conditionally immortalized atrial myocytes with preserved cardiomyogenic differentiation capacity" in Cardiovascular Research, with Jia Liu and Twan de Vries as very proud first author and last author, respectively.
For inquiries please contact us at AM-1@hartlongcentrum.nl
The abstract of our paper reads as follows.
Aims: The generation of homogeneous cardiomyocyte populations from fresh tissue or stem cells is laborious and costly. A potential solution to this problem would be to establish lines of immortalized cardiomyocytes. However, as proliferation and (terminal) differentiation of cardiomyocytes are mutually exclusive processes, their permanent immortalization causes loss of electrical and mechanical functions. We therefore aimed at developing conditionally immortalized atrial myocyte (iAM) lines allowing toggling between proliferative and contractile phenotypes by a single-component change in culture medium composition.
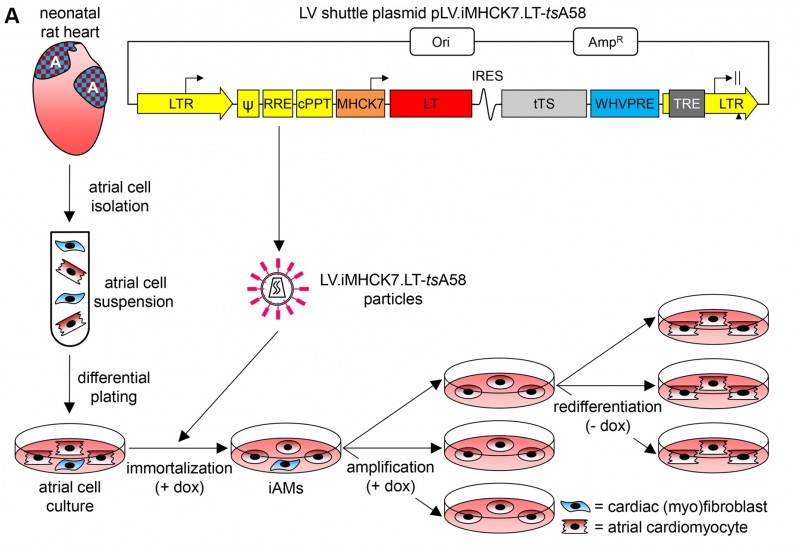
Methods and results: Freshly isolated neonatal rat atrial cardiomyocytes (AMs) were transduced with a lentiviral vector conferring doxycycline-controlled expression of simian virus 40 large T antigen. Under proliferative conditions (i.e. in the presence of doxycycline), the resulting cells lost most cardiomyocyte traits and doubled every 38 hours. Under differentiation conditions (i.e. in the absence of doxycycline), the cells stopped dividing and spontaneously reacquired a phenotype very similar to that of primary AMs in gene expression profile, sarcomeric organization, contractile behaviour, electrical properties and response to ion channel-modulating compounds (as assessed by patch-clamp and optical voltage mapping). Moreover, differentiated iAMs had much narrower action potentials and propagated them at >10-fold higher speeds than the widely used murine atrial HL-1 cells. High-frequency electrical stimulation of confluent monolayers of differentiated iAMs resulted in reentrant conduction resembling atrial fibrillation, which could be terminated by tertiapin treatment, just like in monolayers of primary AMs.
Conclusion: Through controlled expansion and differentiation of AMs, large numbers of functional cardiomyocytes were generated with properties superior to the differentiated progeny of existing cardiomyocyte lines. iAMs provide an attractive new model system for studying cardiomyocyte proliferation, differentiation, metabolism and (electro)physiology and to investigate cardiac diseases and drug responses, without using animals.
This study received financial support from the Netherlands Heart Institute , the Royal Netherlands Academy of Arts and Sciences and Ammodo. Jia was supported by the Chinese Scholarship Council.