Balazs has published his study entitled "The Effects of Repetitive Use and Pathological Remodeling on Channelrhodopsin Function in Cardiomyocytes" in a special issue of Frontiers in Physiology dedicated to cardiac optogenetics.
Abstract:
Aim: Channelrhodopsins (ChRs) are a large family of light-gated ion channels with distinct properties, which is of great importance in the selection of a ChR variant for a given application. However, data to guide such selection for cardiac optogenetic applications are lacking. Therefore, we investigated the functioning of different ChR variants in normal and pathological hypertrophic cardiomyocytes subjected to various illumination protocols.
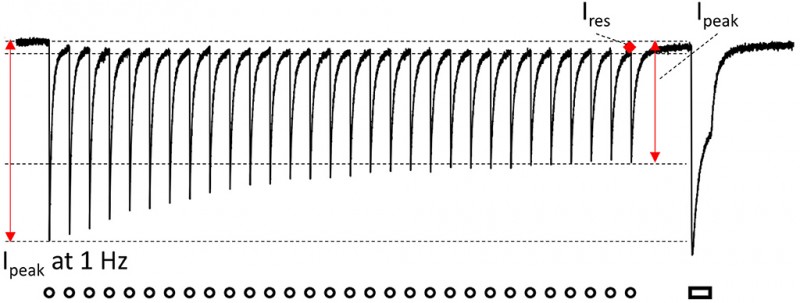
Methods and Results: Isolated neonatal rat ventricular cardiomyocytes (NRVMs) were transduced with lentiviral vectors to express one of the following ChR variants: H134R, CatCh, ReaChR, or GtACR1. NRVMs were treated with phenylephrine (PE) to induce pathological hypertrophy (PE group) or left untreated [control (CTL) group]. In these groups, ChR currents displayed unique and significantly different properties for each ChR variant on activation by a single 1-s light pulse (1 mW/mm2: 470, 565, or 617 nm). The concomitant membrane potential (Vm) responses also showed a ChR variant-specific profile, with GtACR1 causing a slight increase in average Vm during illumination (Vplateau: −38 mV) as compared with a Vplateau > −20 mV for the other ChR variants. On repetitive activation at increasing frequencies (10-ms pulses at 1–10 Hz for 30 s), peak currents, which are important for cardiac pacing, decreased with increasing activation frequencies by 17–78% (p < 0.05), while plateau currents, which are critical for arrhythmia termination, decreased by 10–75% (p < 0.05), both in a variant-specific manner. In contrast, the corresponding Vplateau remained largely stable. Importantly, current properties and Vm responses were not statistically different between the PE and CTL groups, irrespective of the variant used (p > 0.05).
Conclusion: Our data show that ChR variants function equally well in cell culture models of healthy and pathologically hypertrophic myocardium but show strong, variant-specific use-dependence. This use-dependent nature of ChR function should be taken into account during the design of cardiac optogenetic studies and the interpretation of the experimental findings thereof.
For this issue, Tobias Brügmann (Institute of Cardiovascular Physiology, University Medical Center Göttingen, Germany), Stephan Lehnart (University Medical Center Göttingen, Germany), and Godfrey Smith (University of Glasgow, United Kingdom) acted as topic editors. Thank you for inviting us and thank you Robert Kass (Columbia University, USA) and Claudia Richter (Deutsches Primatenzentrum, Germany) for reviewing our paper.